Products
Spine
Sacrum.
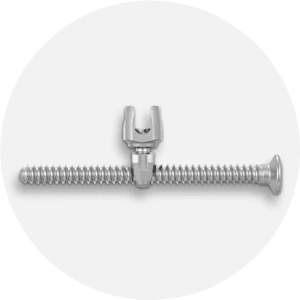
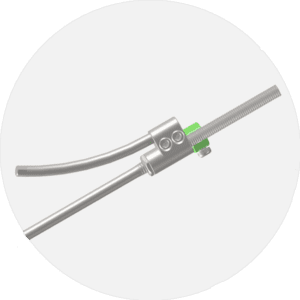
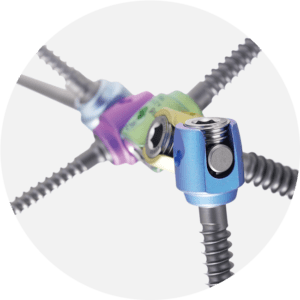
E.SPINE® TANIT®
Iliosacral fixation.
The ilio-sacral fixation E.SPINE® TANIT® is an anchorage device to the pelvis. It is used by minimally invasive posterior approach. The trajectory of the percutaneous ilio-sacral screw is controlled thanks to the guide provided with the ancillary kit. The small size of the implant and its mechanical resistance allow its use in indications of pediatric scoliosis, adult deformities, but also in cases of revision requiring a stabilization of the pelvis. The new TANIT® range offers great intraoperative adaptability thanks to the 3 types of connection (open, closed, reduction) and the two insertion depths of the connector inside the sacrum.
details
• Iliosacral connector enabling 40° range.
• Iliosacral screw available in 17 length.
• Available sterile.
• Notice implant.
• Notice instrument.
• Assembly/disassembly instruction. [1]
Thoracolumbar.
Nemost
Spinal growth osteosynthesis implant.
The NEMOST implant is a sterile, single-use device, intended to be surgically implanted via a posterior approach.
It is a growth implant intended for pediatric use in children with RISSER 0 and as first intention surgery.
The desired effect is a regular lengthening of the device to obtain a gradual correction of the spinal deformity over time while allowing the continued growth of the child.
The NEMOST implant should make it possible to reduce or even eliminate iterative lengthening surgeries of conventional growth constructs.
details
• Automatic one-way lengthening system
• Passive Mechanism: Does not require the use of external activation means
• Simple mechanism with few moving sub-components
• Biocompatible materials (TA6V, PEEK)
• MRI and CT Scan compatible
• Available in 2 elongation sizes: 50mm and 80mm
• Can be used with 5.5mm diameter rods
E.SPINE®
Thoracolumbar sacral osteosynthesis.
The E SPINE® posterior thoracolumbar instrumentation is a compact system designed to treat simple degenerative pathologies up to the most complex spinal deformities. The system provides a unique dual function mono/polyaxial pedicle screw, a variety of rod stiffness and multiple fixation options to the spine and pelvis.
details
• 21 hooks references.
• Pedicle screw available in 5 diameter and 6 length.
• Straight or pre-bent rods.
• Mono/poly axial screws allowing 40° adjustment.
• Supplied non-sterile.
• Implant instruction.
• Instrument instruction.
• Assembly/disassembly instruction. [1]
E.Cross-A
ALIF interbody cage.
E-CROSS-A lumbar cage, made of PEEK, offers a wide choice of vertebral endplates contact surfaces, multiple lordotic angles as well as versatility regarding approach angles. These features make it a cage of choice for all anterior and oblique spine approaches.
details
• lordotic slope up to 20°.
• 30 references across 4 heights.
• Usable as a standalone product thanks to screws across the adjacent vertebral endplates.
• Specific ancillary composed of long instruments to prepare surgery.
• E.ACCESS suitable.
• Graft space.
• Self-tapping screw.
• Supplied sterile.
• Implant instruction.
Custom-made.
Crown
Custom-made plate for cranial reconstruction.
CROWN is a custom-made plate for cranial reconstruction made of PEEK. These implants are design from CT data for a better anatomic adaptation. The plate can be fixed with screws or trans osseous sutures.
details
Hip
Stems.
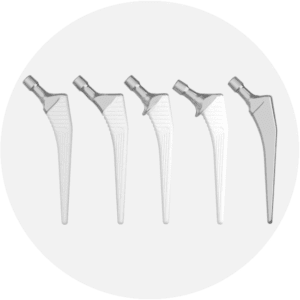
PLM
Cemented or cementless femoral stem.
PLM is a straight self-locking femoral stem. It is available cemented or cementless. It has been designed for primary treatment of hip joint replacement.
details
• TA6V ELI + HAP coating for the cementless version and INOC M30NW for the cemented version.
• Cervico-diaphyseal angle : 135°
• Proximal flaring ensuring tri-axial metaphyseal locking.
• Anti rotating longitudinal grooves.
• Gripping holes to ease implant positioning.
• Thinned polished neck increasing prosthetic hip range of movement.
• 8 standard and lateralised sizes + 3 dysplasic.
• Implant instruction.
• Instrument instruction.
EUROSTEM
Cemented or cementless femoral stem.
The EUROSTEM implant is a quadrangular femoral stem with trapezoidal proximal section and a conical distal section inspired by the CORAIL® philosophy and dedicated to hip arthroplasty.
The EUROSTEM range is available in 4 cementless versions (collarless or collared standard offset, collared coxa vara and high offset and 1 collarless standard offset version to be cemented. This hip range is covering all indications for THR and PHR with 1 instruments set for all of hip approaches.
details
• Straight stem, with a quadrangular cross proximal section
• Horizontal proximal grooves and vertical distal grooves [for
• improved stability]
• Fully hydroxyapatite-coated stem for cementless version
• Non-homothetic increase in sizes
• 12 – 14 taper / 5°42’30» angle
• Cervico-diaphyseal angle: 135°, except for coxa-vara version:
• 125°
• Threaded extraction hole
Acetabulum.
EUROSCUP
Mobile cupula .
EUROSCUP FIXE and EUROSCUP MOBILE implants are a complete range of single mobility and dual mobility acetabular cups. This product range covers all THA indications with 1 instruments set.
The EUROSCUP Range is available in 6 versions: 2 Simple Mobility: EUROSCUP FIXE with PE insert and with ceramic insert and 4 dual Mobility with PE liner: EUROSCUP MOBILE cementless, cemented, tripod and reconstruction.
details
FIXE version
• Hemispherical acetabular cup range made of Titanium and dual coating: Titanium spray and HAP
• Acetabular cups featuring 3 screw holes on upper part for optional Ø6,5mm cancellous screws
• Plugs for polar hole and screw holes
• Tapered connection for ceramic liner enabling easy setting up and extraction
• PE liner clipped into the cup
• Anti-rotation fins on PE liner
MOBILE version
• Cylindro-spherical acetabular cup range divided into 4 versions:
o 1 cemented version made of Cobalt Chromium
o 3 cementless versions made of Cobalt Chromium and dual coating: Titanium spray and HAP.
• Equatorial press-fit.
• Internal groove for cup impaction in the acetabulum
• Internal surface mirror polished
• Cylindro-spherical internal surface to limit the constraints between the head and the liner (piston effect)
• PE liners:
o Ø22 for cups from size 42 to 50mm.
o Ø28 for cups from size 46 to 66mm.
• Tripode version with two impacted pegs and upper screw (Ø6,5mm cancellous screw)
• Reconstruction version with obturator hook and two anchoring flanges for Ø6,5mm cancellous screw
COSYX
Acetabular reinforcement for cemented cup.
COSYX is an acetabular reinforcement system for cemented cups. It allows to cover revision indications with loss of bone substance as well as primary treatment. Combined with bone graft, COSYX enables reconstruction of severe bone loss substance.
details
• Right and left side within a unique implant t diminish stocks.
• One inferior branch in the shape of a hook placed in the obturator foramen.
• Two lateral branches positioned in the anterior and posterior section of the acetabular cavity.
• Stability ensured assembly using 4 cancellous screws of Ø 6.5 mm in the superior branch.
• External Ø 54mm to 70mm.
• Implant instruction.
• Instrument instruction.
Cephalic implants (Partial Hip Replacement).
MOBICUP
Mobile cupula .
MOBICUP is a mobile cupula for restauration of hip mobility. It has been designed for primary treatment of partial hip replacement. It restores hip mobility.
details
• 5/8th of mirror-polished sphere.
• Polytethylene part supplied with removable retention ring.
• Two gripping holes in the retention ring to ease implant positioning.
• Ø 41mm to 59mm range with Ø 28mm inner diameter.
• Implant instruction.
• Instrument instruction.
ONE HEAD
Unipolar modular prosthesis.
ONE HEAD is a cephalic prosthesis offering a simple, quick and affordable solution to treat femoral neck fracture. It restores hip mobility. It restores hip mobility.
details
• Femoral unipolar head with female taper fitted with 3 raised ridges 120° apart.
• Lightweight due to the hollow interior.
• Sleeve designed with 9 grooves 120° apart, three positions can be chosen: Short neck position (-4 mm), Medium neck position (+0 mm), Long neck position (+4 mm).
• Unique sleeve allowing stock reduction.
• Minimal and easy to use instruments consisting of one tray.
• Ø 42mm to 58mm range.
• Implant instruction.
• Instrument instruction.
Knee
Unicompartmental.
EUROP UNI FIXE
Cemented or cementless unicompartmental knee prosthesis.
EUROP UNI FIXE is a Cemented or cementless unicompartmental knee prosthesis designed for partial knee joint replacement. It is available in TAV6/PE of full PE version.
details
• Anatomical femoral condyle : Medial right, Medial left and Lateral.
• Low condyle thickness to maximize preservation of bone stock.
• 2 raising pegs that ensuring stability.
• Two anti-rotating sagittal pins ensuring tibial baseplate stability.
• Possibility to add one screw with the metal baseplate.
• Three radiopaque controls to check the correct positioning of the full PE insert perioperatively and postoperatively.
• Implant instruction.
• Instrument instruction.
• Assembly/disassembly instruction [1][2]
Tricompartmental.
EUROP MOBILE
Asymmetrical mobile bearing knee prosthesis.
EUROP MOBILE is an asymmetrical mobile bearing knee prosthesis cemented or cementless designed for partial knee joint replacement. It is a highly congruent prothesis with or without conservation of the PCL (Posterior Crucial Ligament).
details
Femoral condyle :
• Asymmetric posterior condyles.
• Constant radius of curvature of the trochlear groove to prevent femoro-patellar impingement.
Tibial baseplate :
• Posterior notch respecting the insertion of the PCL.
• Stabilized by 2 anti-rotation wings.
• Extension Keel available.
Tibial insert :
• Posterior notch respecting the insertion of the PCL.
• Anterior notch avoiding conflict with patellar tendon.
• Highly congruent insert ensuring posterior stabilisation.
• Conical fitting allowing free rotation.
• Implant instruction.
• Instrument instruction.
• Assembly/disassembly instruction [1][2][3][4]
HEXAGON
Total Knee Prosthesis – A complete range, from ultra-congruent to posterior-stabilized, with fixed or mobile bearing
HEXAGON is a tricompartmental total knee prosthesis, available in fixed or mobile bearing designs, with ultra-congruent or posterior-stabilized configurations, and with or without cement.
It is designed to address the full spectrum of primary knee arthroplasty indications, while respecting the physiological joint kinematics and ensuring long-term stability.
details
Femoral condyle :
Sagittal profile with three radii of curvature, reproducing the physiological knee kinematics.
Wide flexion range, allowing patients to resume high-flexion activities.
Anatomical trochlea with a 5° angle, reducing the risk of patellar maltracking and overpressure.
Distal and posterior cuts identical (8 mm) for optimal balance of joint spaces.
Available in ultra-congruent and posterior-stabilized versions, depending on the surgical indication.
Tibial baseplate :
Symmetrical design, usable on both right and left sides.
5° posterior slope promoting tibial bone integration.
140° fins with keel shape ensuring optimal anti-rotational stability.
Compatible with an optional extension keel through a screwed, conical connection.
Fixed tray: anterior and posterior locking mechanisms.
Mobile tray: mirror-polished surface reducing metal/PE friction.
Tibial insert :
Made of high-density UHMWPE.
Available in fixed or mobile versions, standard (ultra-congruent) or posterior-stabilized.
Available thicknesses: 7 to 19 mm, depending on the version.
Patellae:
Made of UHMWPE, available in resurfacing version (8 mm thick with fixation pegs) or buried.
Preserves bone stock and natural patellar kinematics.
Shoulder
Anatomical.
SCULTRA II STANDARD
Anatomical shoulder prosthesis.
SCULTRA II STANDARD is an anatomical shoulder prosthesis designed for shoulder scapulo-humeral replacement as a primary treatment. The diversity of the various elements that compose the prosthetic assembly enables to accurately reproduce joint architecture.
details
• Eccentric ring allowing to offset humeral head.
• Humeral stems provided cemented or cementless in several diameters and two lengths.
• Cemented glenoid implants available in 3 sizes anchored by pegs.
• 6 diameters of humeral heads available.
• 5 references of cones available.
• Implant instruction.
• Instrument instruction.
• Assembly/disassembly instruction.
SCULTRA II REVISION
Anatomical shoulder revision prosthesis.
SCULTRA II REVISION complements the SCULTRA II REVERSE prosthesis. This set is composed of a glenoidal baseplate providing additional anterior anchoring as well as on the coracoidprocess and a humeral lipped head (adjustable height thanks to spacers).
details
• Large head to fill acromion-glenoid space.
• Spacer for muscle tension adjustment.
• Revision baseplate fitted with bendable anchoring tabs. Grafting ready. Grafting ready.
• Holed endplate facilitating bone regrowth.
• 3 stem lengths available.
• Implant instruction.
• Instrument instruction.
• Assembly/disassembly instruction.
Reverse.
SCULTRA II REVERSE
Reverse shoulder prosthesis.
SCULTRA II REVERSE is a reverse shoulder prosthesis designed for shoulder scapulo-humeral replacement as a primary treatment. This prothesis takes over GRAMMONT concept. SCULTRA II REVERSE expands its indications throughout a complete set of revision implants.
details
• Humeral stem in several diameters and two lengths.
• Range of inserts to adjust the height and the congruence.
• The addition of a raising block allows a shift of the insert.
• Graft space within the glenoid baseplate.
• Holed endplate facilitating bone regrowth.
• The 135° neck-shaft angle lateralizes the humerus. It increases the lever arm.
• Adjustable position of the Glenoid sphere to increase lever arm.
• Implant instruction.
• Instrument instruction.
• Assembly/disassembly instruction.